DR. RER. NAT. BJÖRN RISSIEK
Nanobody Development
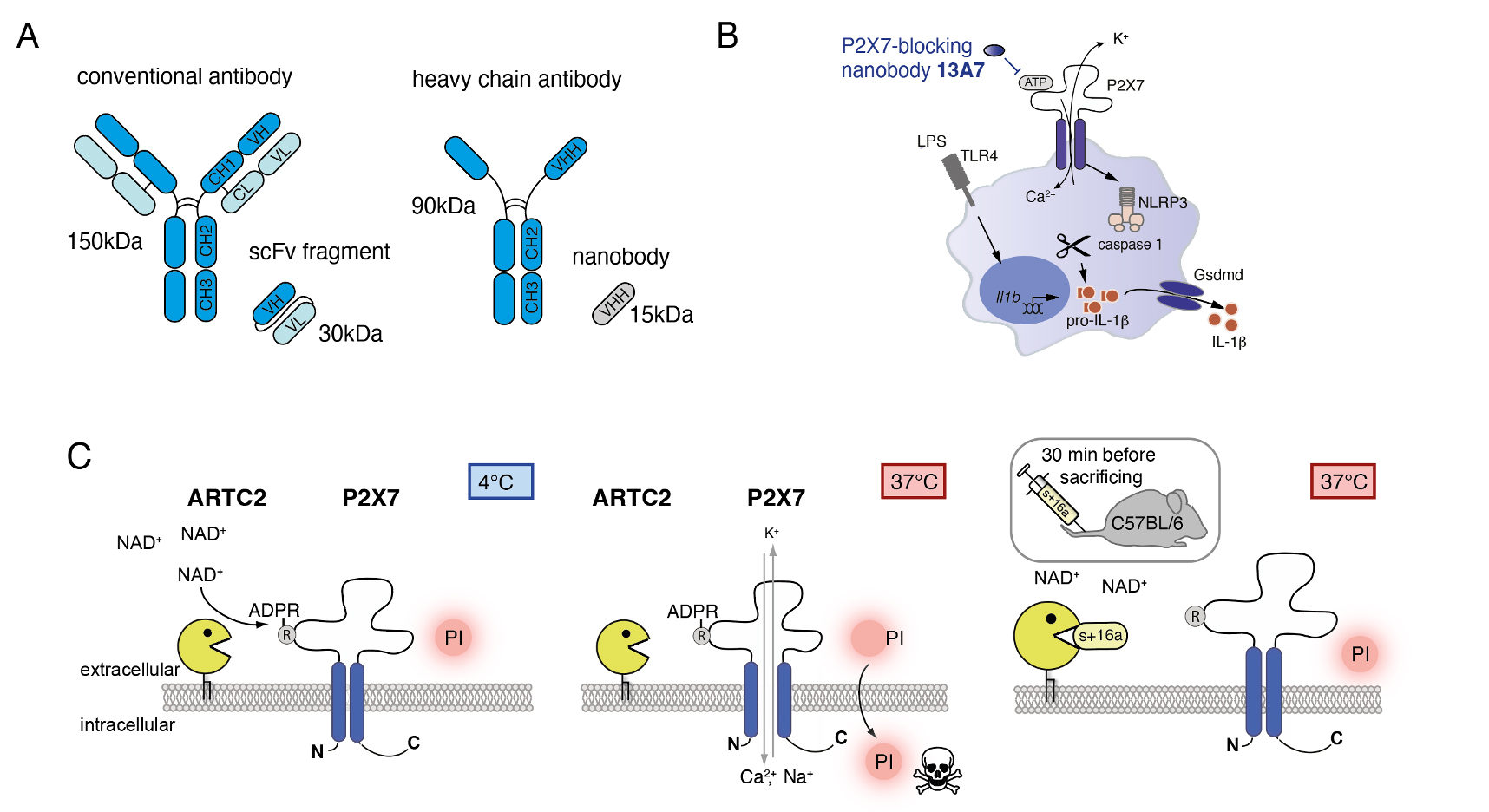
Conventional antibodies, as they exist in all mammalian species, are formed by two heavy and two light chains (Fig.1A). Camelids, such as the llama, have an additional class of antibodies which consists only of two heavy chains, so called “heavy chain antibodies” or in short “HcAbs”. In HcAbs, antigen recognition occurs by binding of a single domain, termed VHH, to the antigen. These VHH domains can be recombinantly expresses as “Nanobodies”, which are 10 times smaller than conventional antibodies and half the size of a single chain variable fragment (scFv). Nanobodies have features similar to conventional antibodies, as they recognize their cognate antigen via binding of the three complementarity determining regions (CDRs). However, CDRs from VHH domains tend to form finger like extrusions, with which they can reach deep into molecular cavities. This makes them excellent antagonist of enzymes or ligand gated cell surface receptors.
The aim of the Nanobody Development Project is to develop new Nanobodies against key signaling molecules of inflammation and immunity. For this we, utilize genetic immunization strategies, immunizing llamas and isolating target-specific Nanobody clones by phage display. In a first step, these Nanobodies are used as tools in preclinical research to study mechanisms of inflammation, with the option of being evaluated in therapeutic settings in clinical studies. As part of SFB1328, Z02 and A13 project generated several different Nanobody clones against the ecto-enzymes CD39 and CD73, which are key players in the degradation of extracellular adenosine triphosphate (ATP) via adenosine diphosphate (ADP) and adenosine monophosphate (AMP) to adenosine. Extracellular ATP is known to act pro-inflammatory e.g. by activating the inflammasome via the P2X7 receptor, followed by the release of interleukin 1 beta (IL-1b). Adenosine, in contrast, conducts anti-inflammatory signals via cell surface adenosine receptors (ADORAs) on various cell types.
In the past, we contributed to the generation and characterization of P2X7-modulating nanobodies developed by in the lab of Prof. Koch-Nolte, UKE Hamburg. Clone 13A7 specifically binds to mouse P2X7 and prevents ATP-induced calcium influx, inflammasome formation and IL-1b release via the Gsdmd pore formation in LPS-primed macrophages (Fig.1B). We also produce the ARTC2.2 blocking Nanobody clone s+16a that is used as tool to enable the isolation of T cell populations sensitive to NAD-induced cell death, such as tissues-resident memory T cells (Trem), NKT cells, Tfh or Tregs (Fig.1C). Collaboration requests can be sent to b.rissiek@uke.de.
Fig.1: Nanobody Development. (A) Structures of conventional antibodies, single chain variable fragments (scFvs), heavy chain antibodies and nanobodies. (B) Nanobody clone 13A7 blocks ATP-mediated activation of the P2X7 receptor, calcium influx, inflammasome formation and release of mature interleukin 1 beta (IL1b). (C) Nanobody s+16a prevents ADP-ribosylation of P2X7 during cell preparation (4°C) and apoptosis, when prepared T cells are brought back to 37°C (adapted from Rissiek et al 2018 Front Immunol).
Selected publications:
Rissiek B, Danquah W, Haag F, Koch-Nolte F. Technical Advance: a new cell preparation strategy that greatly improves the yield of vital and functional Tregs and NKT cells. J Leukoc Biol. 2014 Mar;95(3):543-9.
Rissiek B, Koch-Nolte F, Magnus T. Nanobodies as modulators of inflammation: potential applications for acute brain injury. Front Cell Neurosci. 2014 Oct 21;8:344.
Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, Li J, Davey GM, Kato Y, Devi S, Skandari R, Pauley M, Manton JH, Godfrey DI, Braun A, Tay SS, Tan PS, Bowen DG, Koch-Nolte F, Rissiek B, Carbone FR, Crabb BS, Lahoud M, Cockburn IA, Mueller SN, Bertolino P, McFadden GI, Caminschi I, Heath WR. Liver-Resident Memory CD8+ T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity. 2016 Oct 18;45(4):889-902.
Danquah W*, Meyer-Schwesinger C*, Rissiek B*, Pinto C, Serracant-Prat A, Amadi M, Iacenda D, Knop JH, Hammel A, Bergmann P, Schwarz N, Assunção J, Rotthier W, Haag F, Tolosa E, Bannas P, Boué-Grabot E, Magnus T, Laeremans T, Stortelers C, Koch-Nolte F. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med. 2016 Nov 23;8(366):366ra162. *equally contributing
Rissiek B, Lukowiak M, Raczkowski F, Magnus T, Mittrücker HW, Koch-Nolte F. In Vivo Blockade of Murine ARTC2.2 During Cell Preparation Preserves the Vitality and Function of Liver Tissue-Resident Memory T Cells. Front Immunol. 2018 Jul 9;9:1580.
Jank L, Pinto-Espinoza C, Duan Y, Koch-Nolte F, Magnus T, Rissiek B. Current Approaches and Future Perspectives for Nanobodies in Stroke Diagnostic and Therapy. Antibodies (Basel). 2019 Jan 3;8(1):5.
Bergmann P, Garcia de Paco E, Rissiek B, Menzel S, Dubberke G, Hua J, Rassendren F, Ulmann L, Koch-Nolte F. Generation and Characterization of Specific Monoclonal Antibodies and Nanobodies Directed Against the ATP-Gated Channel P2X4. Front Cell Neurosci. 2019 Nov 12;13:498.
Schriewer L, Schütze K, Petry K, Hambach J, Fumey W, Koenigsdorf J, Baum N, Menzel S, Rissiek B, Riecken K, Fehse B, Röckendorf JL, Schmid J, Albrecht B, Pinnschmidt H, Ayuk F, Kröger N, Binder M, Schuch G, Hansen T, Haag F, Adam G, Koch-Nolte F, Bannas P. Nanobody-based CD38-specific heavy chain antibodies induce killing of multiple myeloma and other hematological malignancies. Theranostics. 2020 Feb 3;10(6):2645-2658.