PD DR. BERTA PUIG MARTORELL
Extracellular vesicles and stroke
The goal of our working group is to understand the mechanisms whereby (1) the EXTRACELLULAR VESICLES (EVs) and (2) the SYNAPTIC DEGENERATION AND RECOVERY play a role in the pathology of stroke.
(1) EVs are nano-sized vesicles released by most of the cells and, although once were thought as being „trash cans“, nowadays are recognized as very important tools of cellular communication. They carry mRNAs, miRNAs, proteins, lipids, and have the ability to elicit a response in recipient cells. They can be of different origins and sizes giving rise to exosomes (≤ 200 nm originated at the endocytic pathway) or microvesicles (≥200 nm and originated from the shedding of the plasma membrane). They are also regarded as promising diagnostic and therapeutic tools for brain diseases because they can cross the blood-brain barrier (BBB).
In our group, we are currently isolating EVs from brain of mice that have been subjected to tMCAO (as an animal model of human stroke), and we use molecular biology tools, advance imaging techniques (confocal and superresolution microscopy), electron microscopy, proteomics, primary cell culture and mRNA-based panels to answer several questions: which are the main populations that release EVs and what is their composition in steady-state and after stroke? If the information that EVs deliver changes after stroke, which are the biological consequences and how this affects the clinical outcome after stroke?
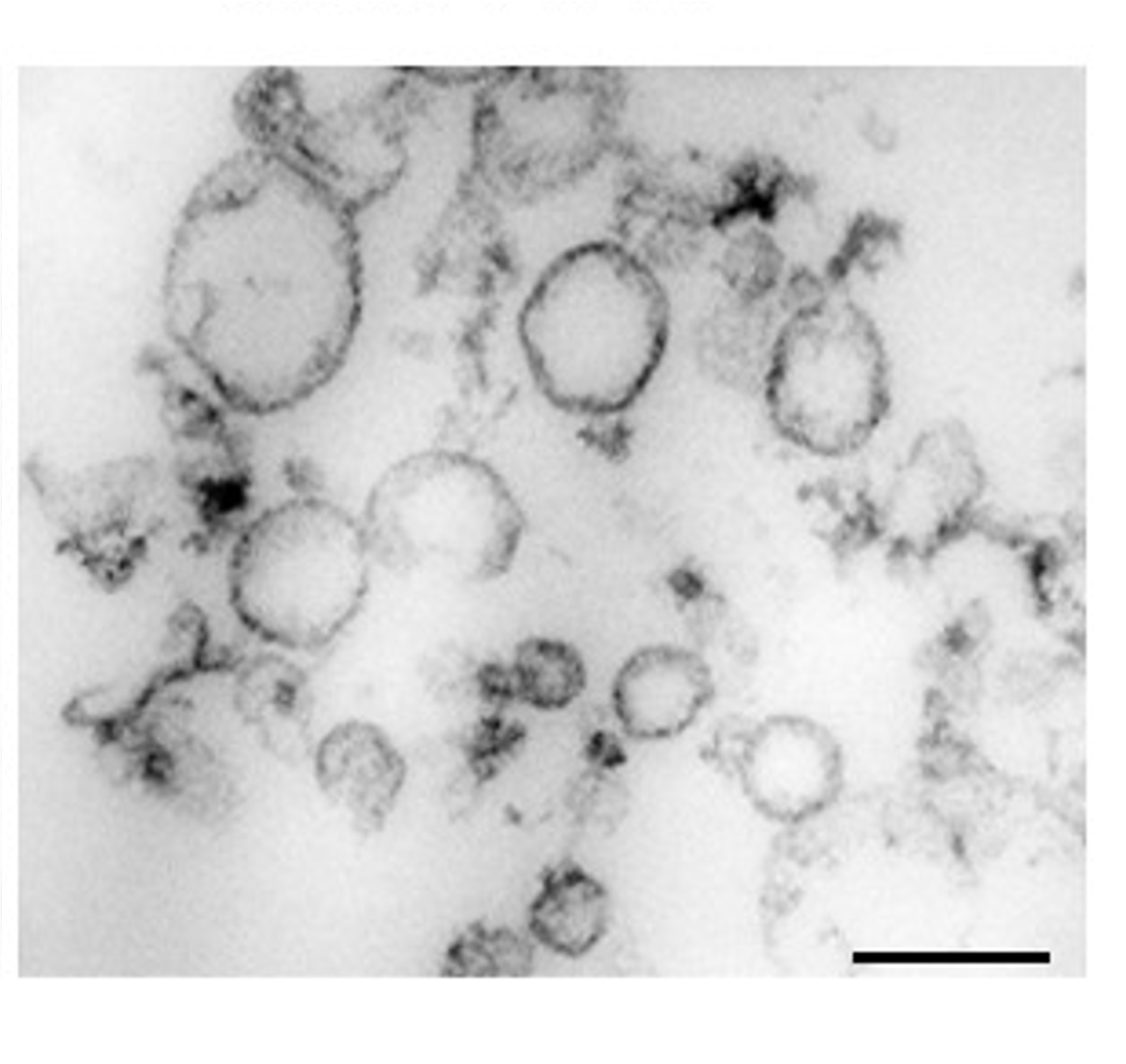
Electron microscopy (EM) picture showing small EVs isolated from mouse brain
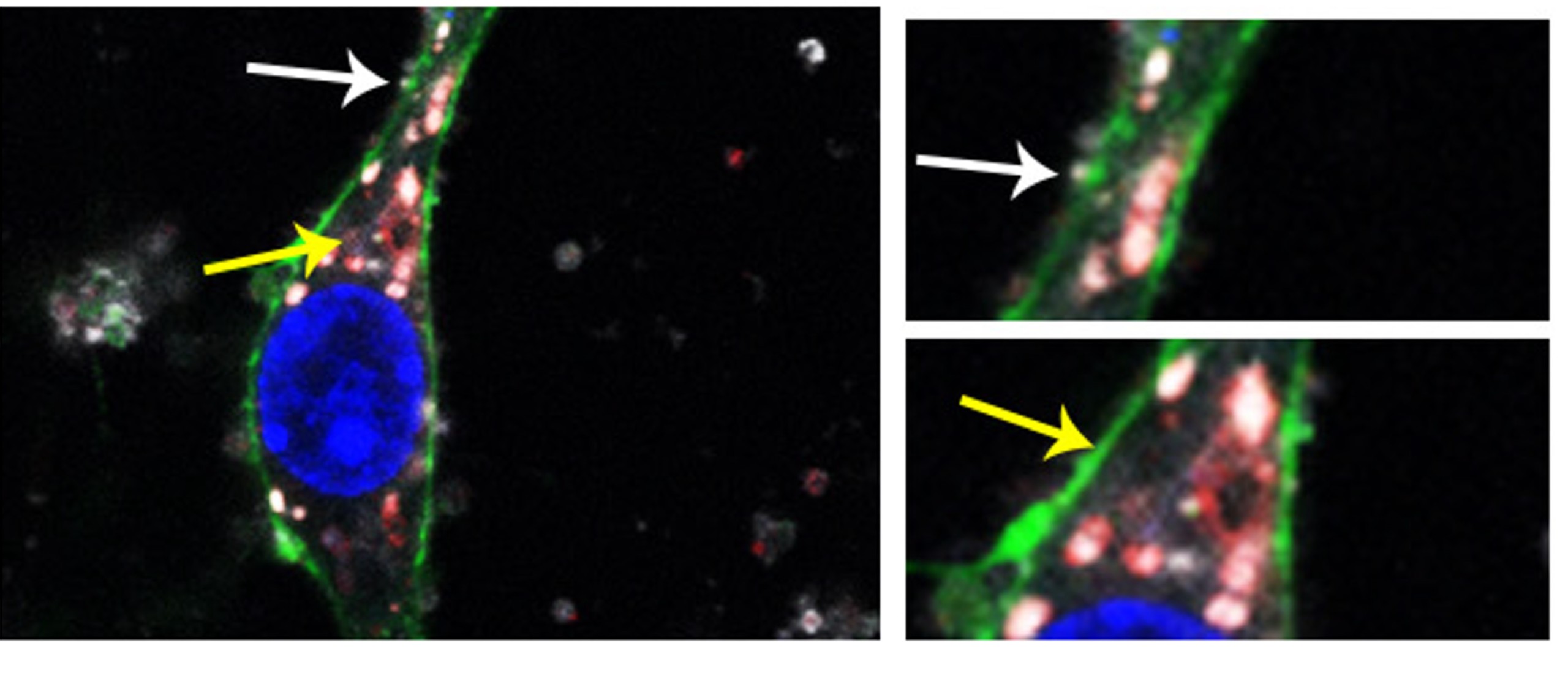
EVs isolated from mouse brain (in white) were incubated with primary neurons (in green). EVs were found colocalizing with lysosomes (labelled in red). White arrows indicate EVs at the membrane (on the way to enter the cell) and yellow arrows, EVs colocalizing with lysosomes
(2) The penumbra is an area surrounding the stroke core where cells, due to the deleterious signals coming from dead cells in the ischemic core, are electrically silent but metabolically active. Their fate to dye or survive will be decided in the next hours or days depending on the stimuli that they receive and can contribute to aggravating the ischemic damage and clinical outcome. Therefore, our goal is to understand through proteomics and molecular biology tools, which are the proteins involved in the SYNAPTIC DEGENERATION AND REGENERATION in the penumbra area in a time-fashion manner, to shed light in the self-recover neuronal mechanism after stroke.