DR. RER. NAT. BJÖRN RISSIEK
DR. RER. NAT. BJÖRN RISSIEK
Cerebral vasculitis
Preclinical research
Key features of brain blood vessel inflammation such as initial triggers, autoimmune mechanisms and immunomodulatory processes during the course of disease are still poorly understood. Since diseases such as PACNS are very rare, the scarcity of available samples from patient suffering from CNS vasculitis might be one major roadblock when it comes to understanding immunopathology and to develop new therapeutic approaches. Retrospectively, preclinical mouse model studies were highly instrumental in understanding disease pathology and the development of new treatment strategies when it comes to other CNS autoimmune diseases, e.g. multiple sclerosis. Therefore, this project aims at developing new mouse model suitable to investigate different aspects of brain blood vessel inflammation. In a first step, we developed the “BrEndO” mouse model. In BrEndO mice, brain (Br) endothelial cells (End) start to express chicken ovalbumin (O) after injection of tamoxifen (Fig.1A). This triggers a CD8+ T cell driven adaptive immune response and leads to the destruction of cerebral blood vessel (Fig.1B). We use the BrEndO mouse model to study cellular aspects of the T cell response against OVA-expressing brain blood vessels and the response of brain endothelial cells towards a T cell attack. Ultimately, we evaluate new therapeutic strategies in the BrEndO mouse model.
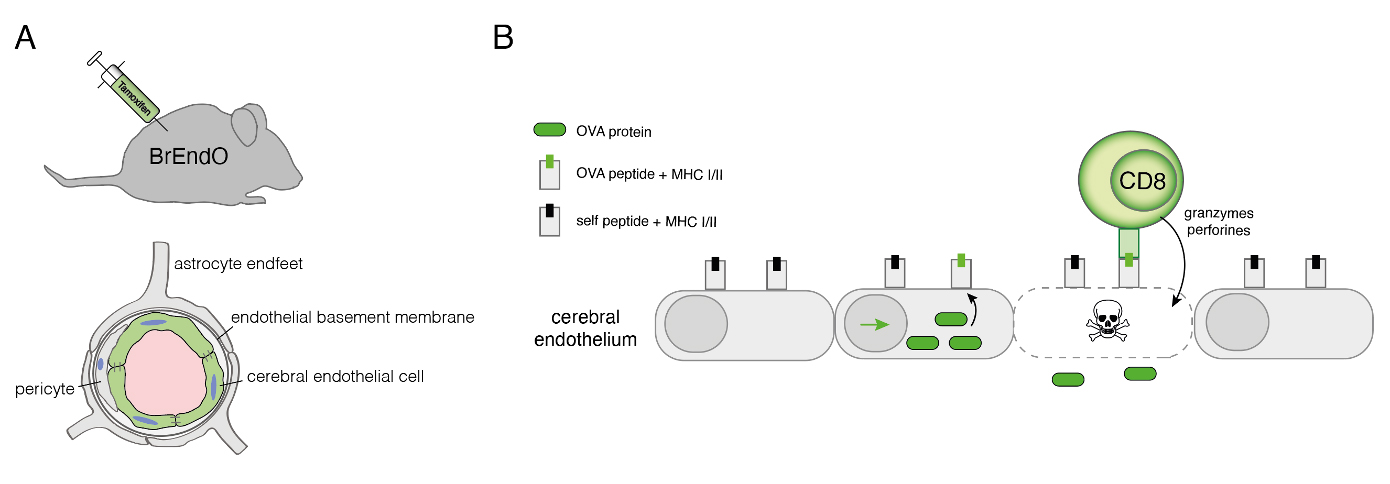
Fig.1: Preclinical mouse models for CNS vasculitis. (A) BrEndO mice express OVA in brain endothelial cells after injection of Tamoxifen. (B) CD8 T cells recognize OVA peptides presented in MHC class I with the consequence of endothelial cells being destroyed.
Clinical research
Primary angiitis of the central nervous system (PACNS) is an inflammatory, poorly understood disease affecting the small- and medium-sized vessels of the brain and spinal cord. All age groups may be affected. Given that PACNS can also be a cause of stroke, in particular, it remains an important differential diagnosis in younger (<45 years) adults.
The diagnosis of PACNS is extremely hampered by the paucity of specific clinical symptoms and by the low specificity of the diagnostic approaches. At present, brain biopsy is the only eligible technique to establish a definite diagnosis of PACNS. But, even this gold-standard procedure is hallmarked by a low sensitivity, resulting in a high rate of patients with a highly suspected PACNS compared to their counterparts, the biopsy-proven patients.
Thus, establishing a reliable diagnosis remains challenging and PACNS is often misdiagnosed. Moreover, it is important to exclude other diseases which bear the closest resemblance to PACNS such as reversible cerebral vasoconstriction syndrome (RCVS) or moyamoya disease (MMD), both representing non-inflammatory vasculopathies. However, the potential aggressive course of the disease necessitates a high diagnostic accuracy for safely instituting the required immunosuppressive treatment.
This project focuses on new diagnostic tools, such as potential biomarkers in PACNS which facilitate the diagnostic workup. Our key scientific interest is to identify a panel of biomarkers for PACNS that increase the certainty of the diagnosis.
We already identified the circulating endothelial cells (CECs) and as a novel approach the endothelial progenitor cells (EPCs) in the peripheral venous blood as well as interleukin 17 (IL-17) in the cerebrospinal fluid as very promising diagnostic markers and markers for disease activity in PACNS. In line with our clinical research approaches so far, we established a first data base for PACNS patients with histological evidence. We intend to further expand this data base. Furthermore, we participate in the first international, prospective multi-center registry for systemic and CNS vasculitis (GeVas), in which we are the leading coordinators for CNS vasculitis/PACNS.